A bilirubin nanoparticle is made of poorly soluble bilirubin core and hydrophilic PEG shell. The nature of bilirubin allows anti-cancer drugs to be easily encapsulated in the nanoparticle via hydrophobic interaction.
Although there are many anti-cancer drugs available, patients are still suffering from side effects due to drug toxicity. Our BRIXELLE-ONCO technology focuses on alleviating patients’ suffering by minimizing side effects by utilizing bilirubin nanoparticles as non-toxic drug carrier. Our bilirubin nanoparticle is synthesized by conjugating well validated PEG to the bilirubin molecule. As bilirubin naturally exists in the body and safety of PEG is already well known, the bilirubin nanoparticle itself possesses no toxicity.
The bilirubin nanoparticle is approximately 100nm in size where the inner core is hydrophobic and the surface layer of PEG is hydrophilic. The technology is based on simply loading hydrophobic anti-cancer agents into the core of the bilirubin nanoparticle. Therefore, there is no chemical modification of the anti-cancer agent. Once the drug-loaded bilirubin nanoparticles are administered into the body, the nanoparticles will accumulate in the tumor tissue due to Enhanced Permeability and Retention (EPR) effect. The anti-cancer drug can be released at the tumor site once the bilirubin nanoparticles break down into PEGylated biliverdin(hydrophilic) once the nanoparticles reacts with ROS released from the tumor site.
Increased efficacy through specific distribution to cancer tissues
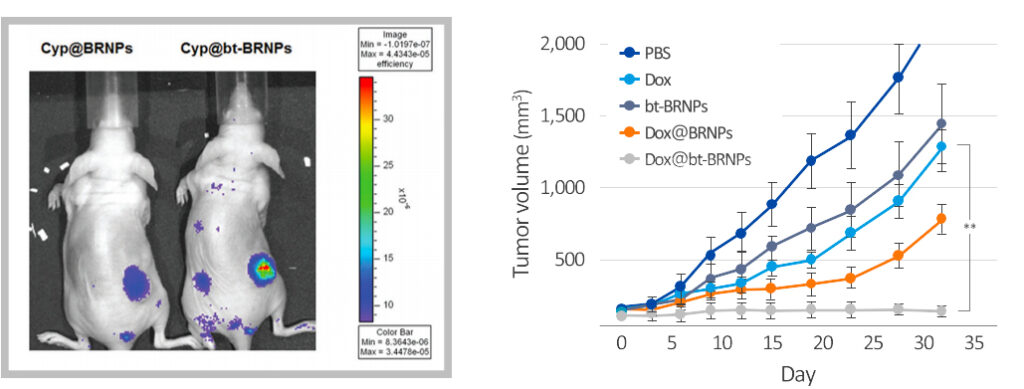
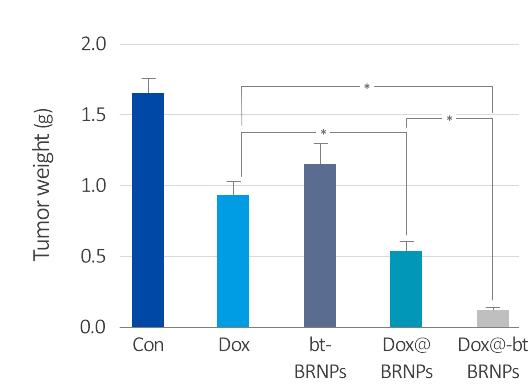
The figure shows that the bilirubin nanoparticles (Brixelle) are well accumulated at tumor sites by enhanced permeability retention effect and improved anticancer activity (decreased tumor volume and tumor weight) in vivo when intravenously administered to mice bearing Hela tumors.
Adv Sci. 2018;5:1800017.
Increasing the effectiveness by increasing the half-life in the body

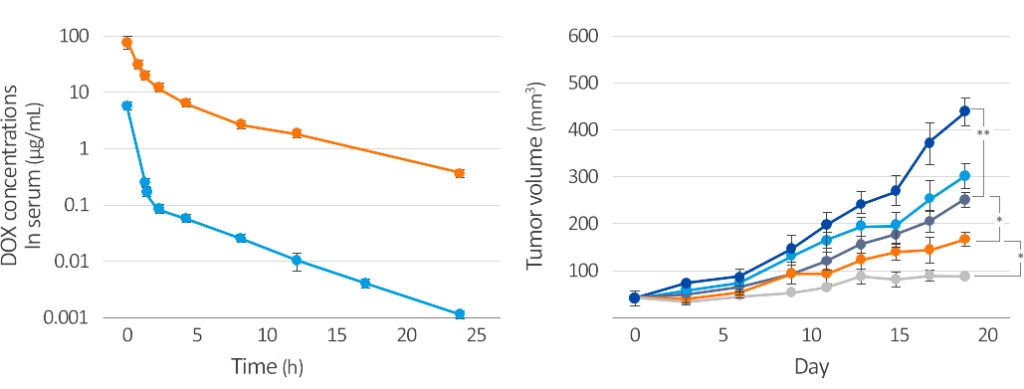
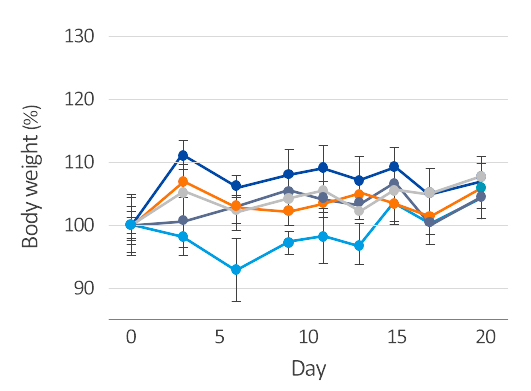
The figure shows that the bilirubin nanoparticles (Brixelle) increase the half-life and Cmax of anti-cancer drugs and improved anticancer activity
Angewandte Chemie Int Ed. 2017;56:13684–13688.
(decreased tumor volume and less bodyweight loss) in vivo when intravenously administered to mice bearing Hela tumors.