Bilirubin and its therapeutic effects in various diseases have long been studied for the past 35 years, resulting in over 27,000 journal articles. However, it could not be therapeutically developed into drugs due to its highly hydrophobic properties, which in turn, causes tissue accumulation and toxicity. Many scientists have tried in previous years to make bilirubin soluble, but failed. Notably, Dr. Phillip Hench observed in 1930s at Mayo Clinic that bilirubin relieved symptoms of rheumatoid arthritis. He then used bilirubin in his clinical trials but could not induce increase in serum bilirubin level. Later, he discovered steroid and was awarded a Nobel Prize in 1950. Dr. Fritz Bach, a renowned surgeon at Harvard University, spent his career to develop bilirubin as therapeutic agent, but could not succeed either.
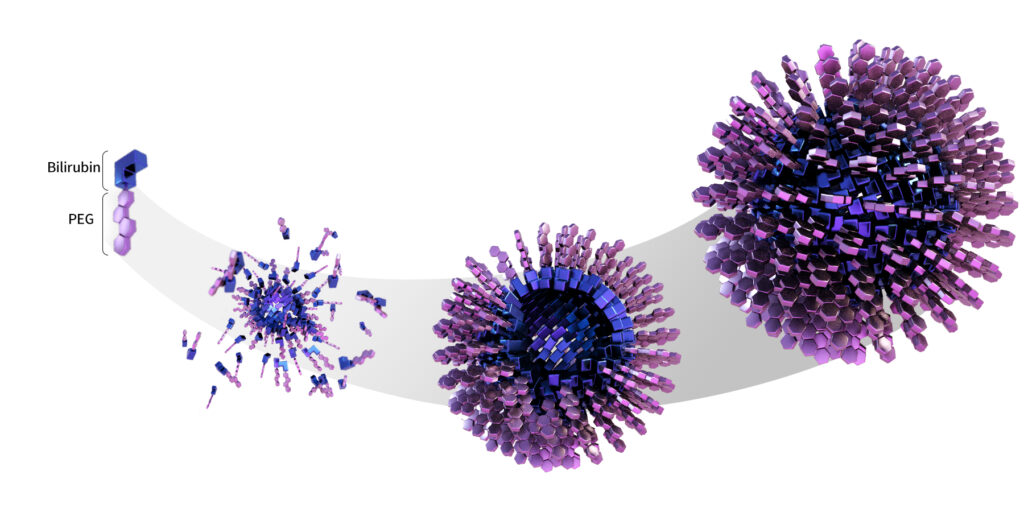
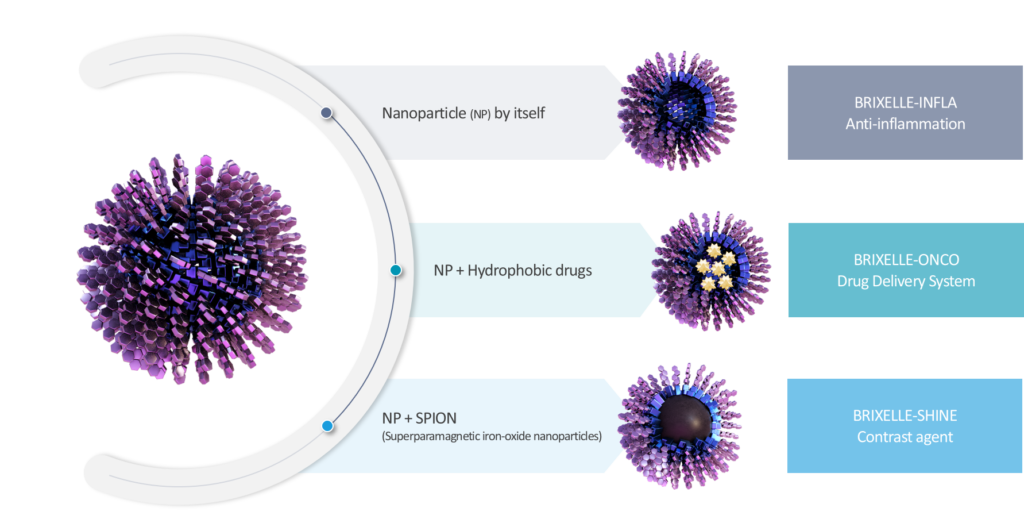
Our technology revolves around chemically synthesized PEGylated bilirubin nanoparticles where the hydrophilic polyethylene glycol(PEG) is covalently bound to the carboxyl group of bilirubin. Conjugation of PEG to any other site of bilirubin would abolish efficacious effects of bilirubin as ROS (Reactive Oxygen Species) scavenger and as immune modulatory molecule. By PEGylating carboxyl group of bilirubin, PEG provides hydrophilicity whilst bilirubin still retains hydrophobicity, rendering the conjugated compound to self-assemble as a micelle in aqueous solution. This is the only way to make bilirubin soluble in water while retaining its own therapeutic effects.
In addition, bilirubin has been commercially available only as porcine-derived material since it has never been chemically synthesized. Very reactive vinyl groups at both ends of bilirubin make it almost impossible to chemically synthesize. Due to such challenges, many scientists have failed to transform this molecule into a therapeutic agent. Our scientist team was able to overcome various steps of synthesis and finally was successful in chemical synthesis for the first time in the world. Our synthetic schemes are so efficient that both the yield and cost effectiveness are comparatively competitive than isolating and purifying from animal derived bilirubin.
Furthermore, when PEGylated bilirubin forms micelle in solution, it can naturally harbor and retain any compound nearby in such a manner that the more hydrophobic the nearby compound is, the better. We used this viable characteristic to encapsulate chemotherapy agents such as Doxorubicin and Cisplatin (data published in 3 journal articles). Suddenly, the same PEGylated bilirubin nanoparticles that are used to treat intractable inflammatory diseases, now can be used to deliver chemotherapy agents to the specific site of tumor as drug delivery system (DDS). Most of marketed DDS either have toxicity or low efficacy since their encapsulating materials do not usually breakdown to release the chemo agents nearby the tumor sites. As a result, aside from removing bilirubin’s known toxicity, we have successfully achieved the following to make our bilirubin nanoparticles into a viable drug candidate.
- Chemically synthesized
- Safety profile is achieved as clinical application of PEG is already widely known
- Improved efficacy due to target-specific property of Enhanced Permeability and Retention (EPR) mechanism
- Increased PK profile compared to bilirubin alone
-
arrow_forwardBRIXELLE-INFLA
-
arrow_forwardBRIXELLE-ONCO
-
arrow_forwardBRIXELLE-SHINE